1042
Views & Citations42
Likes & Shares
There have been less than a dozen reported cases of
intracranial extraskeletalmyxoid chondrosarcoma in the literature. We present the youngest case to date with
review of the literature. A 16-year-old
boy initially presented with headaches and vomiting. At outlying hospital, the patient was found a
large tumor in the pineal location with hydrocephalus. He was referred to our
center after an intracranial biopsy following an endoscopic third
ventriculostomywas not conclusive for pathology. He underwent resection at our institution and
the pathology confirmed an extraskeletalmyxoid chondrosarcoma. The patient underwent adjuvant treatment, but
ultimately required shunting for diffuse leptomeningeal disease. The shunt failed due to viscous CSF due to
excessive presence ofmyxoid material. His disease progressed significantly and
developed carcinomatous ascites and expired 16 months after diagnosis.
INTRODUCTION
Case Report
A 16-year-old male
with no past medical history in otherwise normal health developed progressive
headache over 2 months in the right frontal region. These were worse in the
morning, but progressed to headache throughout the day. Additionally, he
developed a few episode of emesis for 4-6 weeks. These symptoms prompted a CT
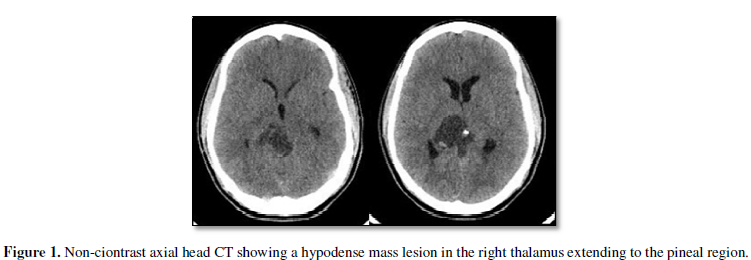
and MRI of the brain at an outside institution. CT brain revealed a hypodense mass with patchy
hyperdense lesion within it in
the right thalamus to pineal region with
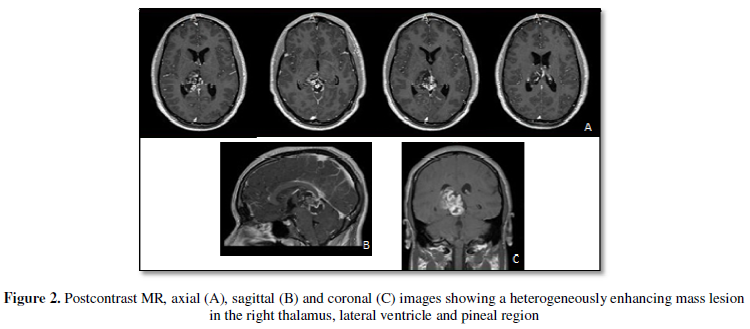
obstructive hydrocephalus (Figure 1). MRI of the brain showed heterogeneous
enhancing lesion extending to the quadrigeminal cistern and lateral
ventricle from the right
thalamus (Figure 2). He had no neurological deficits upon
initial presentation.
The patient underwent endoscopic third ventriculostomy (ETV) and craniotomy at the outside hospital. The
sample had a mucinous mucoid appearance. The pathology was inconclusive as the
craniotomy was aborted due to brain swelling and only biopsy was obtained. His initial
post-operative course was complicated by severe brain edema managed with
induced coma. He made a substantial recovery, but was left with an upper
quadrant hemianopia on the left side and short term memory dysfunction.
Over the next month, the tumor continued to rapidly increase in size
and cross the midline. There was cerebral herniation of the occipital lobe out
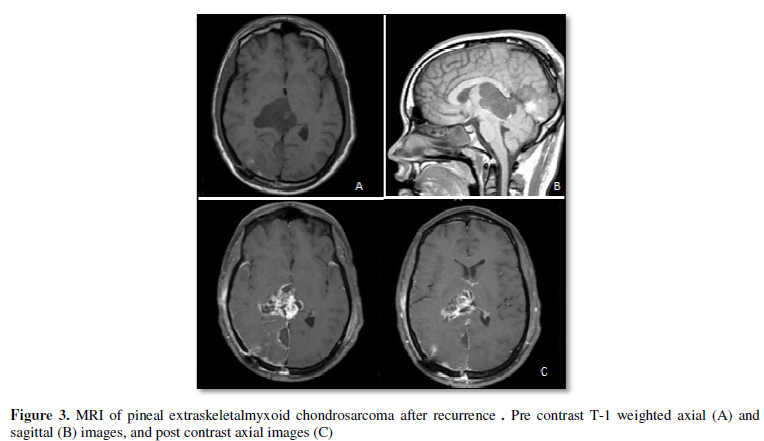
the craniotomy site as well. MR showed contrast enhancing large tumor in the pineal
location extending to the lateral ventricle involving the thalamus of the right
side (Figure 3). His examination
showed a left-side homonymous hemianopia and Parinaud signs. Four months after
the initial craniotomy, he was transferred to our institution for a second
opinion and elected for a repeat craniotomy for further tumor resection. At the
time of surgery an external ventricular drain (EVD) was placed for brain
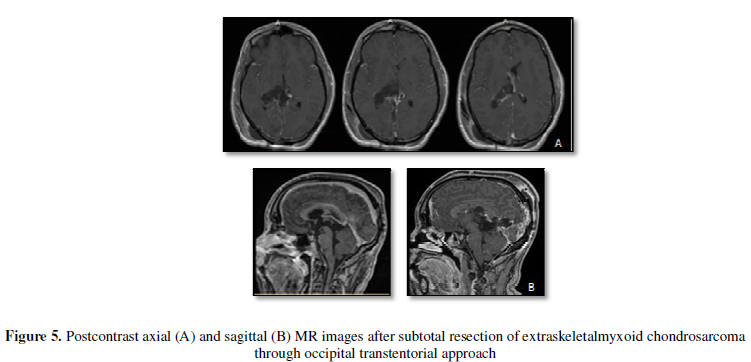
relaxation. He underwent an occipital transtentorial approach for sub-total
resection of the tumor (Figure 4).
Tumor resection was stopped after extensive debulking due to the involvement of
the vein of Galen and internal cerebral veins. Post-operatively he underwent a
ventriculo-peritoneal (VP) shunt placement 1 week after resection due to inability
to wean off the EVD .The CSF profiles at that time showed protein 50 mg/dL,
glucose61mg/dL, 24rbc /cu.mm and 1 wbc/cu.mm without tumor cells. His Parinaud
syndrome eventually resolved.
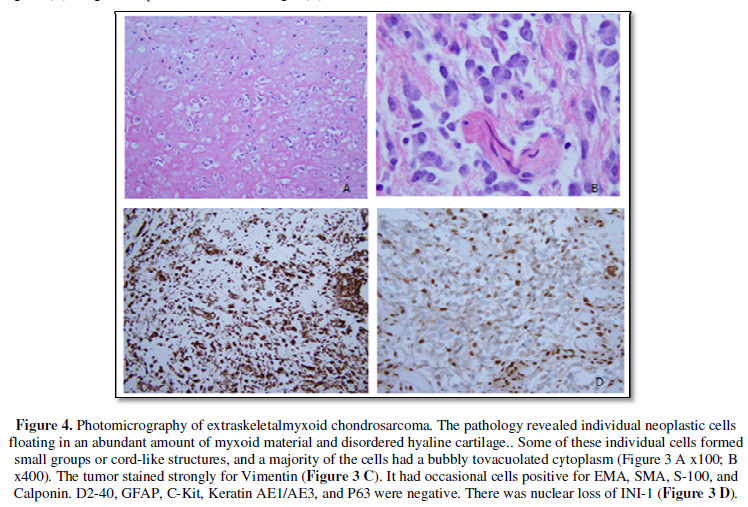
The pathology revealed individual neoplastic cells floating in an
abundant amount of myxoid material. Some of these individual cells formed small
groups or cord-like structures, and a majority of the cells had a bubbly to vacuolated
cytoplasm (Figure 5A, 5B). The tumor stained strongly for Vimentin (Figure 5C). It had
occasional cells positive for EMA, SMA, S-100, and Calponin. D2-40, GFAP,
C-Kit, Keratin AE1/AE3, and P63 were negative. There was loss of normal nuclear
expression of INI-1 (Figure 5D). The differential diagnosis included an
unusual Atypical Teratoid Rhaboid Tumor, Myoepithelial Carcinoma, and an
Extraskeletal Myxoid Chondrosarcoma. Occasional interspersed fragments of dense,
disordered hyaline cartilage with atypical appearing chondrocyte-like cells and
calcification were seen. In conjunction with the above immunohistochemical
stains, the morphology favored an Extraskeletal Myxoid Chondrosarcoma.
He underwent
adjuvant treatment with proton radiation therapy and pazopanib. He received
craniospinal irradiation with boost to the original tumor area due to positive
cerebrospinal fluid (CSF) cytology. He had boost of 30.67 cobalt grey
equivalent (CGE) to the primary site and
36.30 CGE to the craniospinal axis at 2 months postoperatively. However, over the
next 6 months he developed multiple cranial nerve abnormality. By 12 months
postoperatively, he had
decreased vision bilaterally, bilateral hearing loss, facial paralysis, and
uvula deviation. His MRI brain revealed stable lateral ventricle and
thalamic disease with subtle
leptomeningeal enhancement .
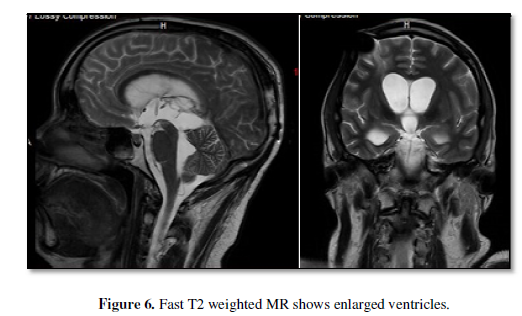
One month later, he
underwent VP shunt exploration after
his lateral ventricles became large and he became poorly responsive (Figure 6). The endoscopic observation showed open ETV but
whitish clumps of tumor floating between the ventricle and prepontinesubarachnoid space, and the ventricular CSF was
extremely viscous (Video 1). The CSF was extremely viscous. The CSF profiles indicate
protein 126 mg/dL, glucose 50 mg/dL, 71 red blood cell /cu.mm and 7 white blood
cell/cu.mm with tumor cells. His
abdomen was not absorbing the fluid causing extensive ascites. The CSF profile 10 days later showed
protein 275 mg.dL, glucose 68 mg/dL, 275 rbc/cu.mm and 52 wbc/cu.mm. Both CSF
and ascites cytology revealed
extensive malignancy. He ultimately received an external pigtail catheter placement into the peritoneum for the ascites and was discharged. He expired 16 months
after the diagnosis.
DISCUSSION
Intracranial extraskeletalmyxoid chondrosarcoma is extremely rare. Extraskeletalmyxoid chondrosarcoma has been described in many organ systems [1,16,17]. Our case appears to have originated from the choroid plexus, thalamus, or the pineal region. A similar case of thalamic location as ours was reported by Park et al. [18], which was considered to have originated from the choroid plexus. However, the extensive tumor invasion makes the origin hard to determine. Our case did not have significant calcification as others have described [16]. The S-100 was focally present as previously described [16,17]. The INI-1 loss is still unclear for the diagnostic utility [19]. However, INI1 negativeextraskeletalmyxoid chondrosarcoma was reported [20].
Importance of total resection was emphasized for tumor control. However,
complete resection is often not possiblefor those occurring in the
cerebellopontine angles, pineal and sellar location. In our case a gross total resection was not attainable
with the extensive invasiveness. Park et al. reviewed the 8 cases reported in
the literature [18]. Six of theseeight were able to have a complete
resection. It appears the
aggressive nature of the tumor warrants an aggressive, but safe surgical plan. Radiation
therapy seems appropriate for such a highly malignant tumor [2,3,5,7,10]. However,
it is unclear about a survival advantage with adjuvant chemotherapy.
At the terminal stage, the patient showed widespread metastasis in the
ventricle and subarachnoid space together with peritoneal cavity through the
shunt.The viscous nature of the ventricular CSF is due to not only theelevated
CSF protein and numerous malignant cells in the CSF but also to the presence of
mucin secreted by myxoidchondrosarcoma.
CONCLUSION
Our case is the youngest reported of an intracranial extraskeletalmyxoid chondrosarcoma. There is a paucity of available data in the literature for these rare tumors. We advocate aggressive maximal safe resection with upfront adjuvant therapy. One should be aware extremely viscous CSF due to presence of myxoid material once the tumor disseminates in the cerebral ventricle.
1. Enzinger
FM, Shiraki M (1972) Extraskeletalmyxoid chondrosarcoma. An analysis of 34 cases.
Hum Pathol 3: 421-435.
2.
Chaskis C, Michotte A, Goossens A,
Stadnik T, Koerts G, et al. (2002) Primary intracerebral myxoid chondrosarcoma.
Case illustration. J Neurosurg 97: 228.
3.
Gonzalez-Lois C, Cuevas C, Abdullah O,
Ricoy JR (2002) Intracranial extraskeletalmyxoid chondrosarcoma: case report
and review of the literature. ActaNeuorochir (Wien) 144: 735-740.
4.
Hero RC, Martinez AJ, Ahn HS (1980)
Intracranial mesenchymal chondrosarcoma. Surg Neurol 14: 311-317.
5.
Im SH, Kim DG, Park IA, Chi JG (2003) Primary
intracranial myxoid chondrosarcoma: report of a case and review of the literature.
J Korean Med Sci 18: 301-307.
6.
Sala F, Talacchi A, Beltramello A,
Izzulino P, Bricolo A (1998) Intracranial myxoid chondrosarcoma with early
intradural growth. J Neurosurg Sci 42: 159-163.
7.
Salcman M, Scholtz H, Kristt D,
Numaguchi Y (1992) Extraskeletalmyxoid chondrosarcoma of the falx. Neurosurgery
31: 344-348.
8.
Sato K, Kubota T, Yoshida K, Murata H
(1993) Intracranial extraskeletalmyxoid chondrosarcoma with special reference
to lamellar inculusions in the rough endoplasmic reticulum. Acta Neuroplathol
86: 525-528.
9.
Scott RM, Dickersin R, Wolpert SM,
Twitchell T (1976) Myxochondrosarcoma of the fourth ventricle. Case report. J
Neurosurg 44: 386-389.
10.
Sorimachi T, Sasaki O, Nakazato S, Koike
T, Shibuya H (2008) Myxoid Chondrosarcoma in the pineal region. J Neurosurg
109: 904-907.
11.
Bourgouin PM, Tampieri D, Robitaille, Y,
Robert F, Bergeron D, et al. (1992) Low-grade myxoid chondrosarcoma of the base
of the skull: CT, MR, and histopathology J Comput Assit Tomogr 16: 268-273.
12.
Cummings TJ, Bridge JA, Fukushima T
(2004) Extraskeletalmyxoid chondrosarcoma of the jugular foramen. Clin Neuropathol
23: 232-237.
13.
Grossman RI, Davis KR (1981) Cranial
computed tomographic appearance eof chondrosarcoma of the base of the skull.
Radiology 141: 403-408.
14.
Hassounah M, Al-Melfty O, Akhtar M,
Jinkins JR, Fox JL (1985) Primary cranial and intracranial chondrosarcoma. A
survey. ActaNeurochir(Wien) 78: 123-132.
15.
Smith TW, Davidson RI (1981) Primary
meningeal myxochondrosarcoma presenting as a cerebellar mass: case report.
Neurosurgery 8: 577-581.
16.
O’Brien J, Thornton J, Cawley D, Farrell
M, Keohane K, et al. (2008) Extraskeletalmyxoid chondrosarcoma of the
cerebellopontine angle presenting during pregnancy. Br J Neurosurg 22: 429-432.
17.
Oliveira AM, Sebo TJ, McGrory JE, Gaffey
TA, Rock MG, et al. (2000) Extraskeletalmyxoid chondrosarcoma: a
clincopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod
Pathol 13: 900-908.
18. Park JH, Kim MJ, Kim JK, Kim JH (2012) Intracranial extraskeletalmyxoid chondrosarcoma: case report and literature review. J Korean Neurosurg Soc 52: 246-249.
19. Korten AG, ter Berg HJ, Spincemaille GH, van der Laan RT, Van de Wel AM (1998) Intracranial chondrosarcoma: review of the literature and report of 15 cases. J NeurolNeurosurg Psychiatry 65: 88-92.
20. Kohashi K, Oda Y, Yamamoto H, Tamiya S, Oshiro Y, et al. (2008) SMARCB1/INI1 protein expression in round cell soft tissue sarcomas associated with chromosomal translocations involving EWS: A special reference to SMARCB1/INI1 negative variant extraskeletalmyxoid chondrosarcoma. Amr J Surgical Pathology 32: 1168-1174.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Pathology and Toxicology Research
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Advance Research on Alzheimers and Parkinsons Disease